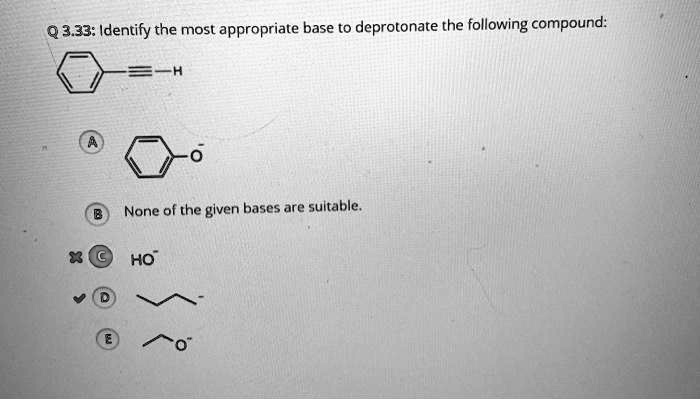
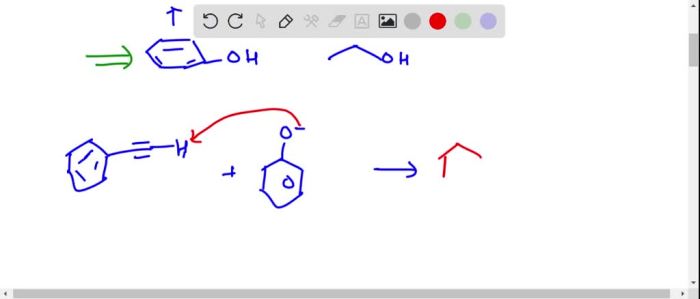
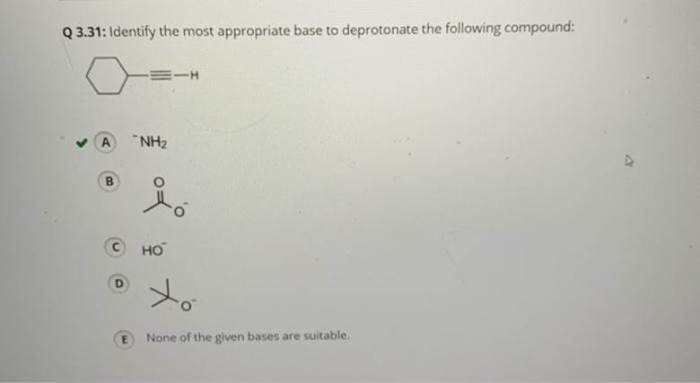
Identify the most appropriate base to deprotonate the following compound: – Deprotonation, the removal of a hydrogen ion from a compound, plays a pivotal role in organic chemistry. To achieve efficient deprotonation, selecting the most appropriate base is crucial. This article delves into the factors influencing base selection, explores common bases used for deprotonation, and discusses considerations for specific compounds and practical applications.
Deprotonation: Base Selection and Considerations
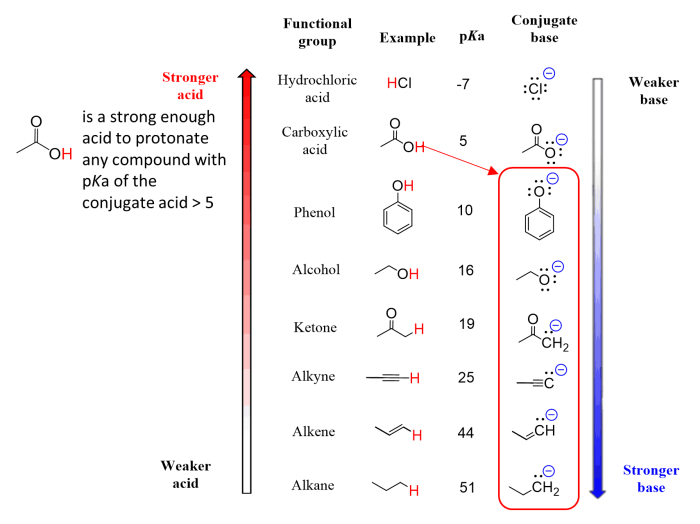
Deprotonation, the removal of a proton (H+) from a molecule, plays a crucial role in organic chemistry. The choice of base for deprotonation is critical for achieving optimal reaction outcomes.
Factors Influencing Base Selection
- Acidity of the Compound:Stronger acids require stronger bases for deprotonation.
- Basicity of the Base:Stronger bases are more effective at deprotonating weaker acids.
- Steric Effects:Bulky bases may encounter steric hindrance, affecting their ability to approach the acidic proton.
- Solvent Effects:The solvent can influence the basicity of the base and the acidity of the compound.
Common Bases Used for Deprotonation
| Name | Formula | pKa | Strengths and Limitations |
|---|---|---|---|
| Sodium hydroxide (NaOH) | NaOH | 15.7 | Strong base, widely used but can lead to over-deprotonation. |
| Potassium tert-butoxide (t-BuOK) | C4H9KO | 18.5 | Strong base with good solubility in organic solvents. |
| Lithium diisopropylamide (LDA) | C6H14LiN | 25.5 | Very strong base, highly reactive and requires low temperatures. |
Considerations for Specific Compounds, Identify the most appropriate base to deprotonate the following compound:
- Alcohols:Weak acids, require strong bases like NaH or LDA.
- Amines:Varying acidity, require bases with appropriate pKa values.
- Carboxylic acids:Relatively strong acids, require bases like NaOH or KOH.
Practical Considerations
- Availability and Cost:Consider the availability and cost of the base.
- Safety and Handling Precautions:Ensure proper safety precautions for handling strong bases.
- Reaction Conditions:Adjust the reaction conditions to optimize deprotonation.
FAQ: Identify The Most Appropriate Base To Deprotonate The Following Compound:
What factors influence base selection for deprotonation?
Factors influencing base selection include acidity of the compound, basicity of the base, steric effects, and solvent effects.
What are some common bases used for deprotonation?
Common bases used for deprotonation include sodium hydride, potassium tert-butoxide, and pyridine.
How do I select the most appropriate base for deprotonating a specific compound?
To select the most appropriate base, consider the acidity of the compound, the basicity of the base, and any steric or solvent effects that may be present.