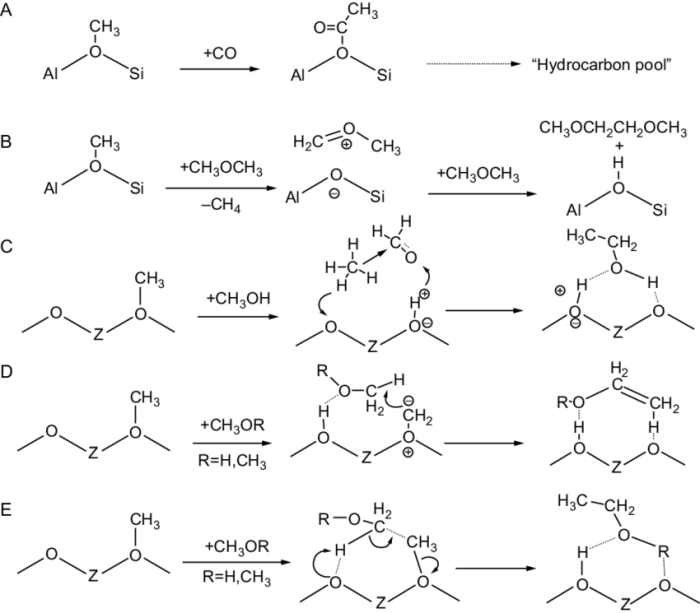
Rank the following compounds in order of increasing water solubility. Water solubility is a crucial property that determines the behavior and applications of various compounds. Understanding the factors that influence water solubility is essential for optimizing their use in different fields.
This guide provides a comprehensive overview of water solubility, ranking criteria, experimental methods, and the implications of solubility differences. By delving into these aspects, we aim to enhance your knowledge and equip you with a deeper understanding of this fundamental property.
Water Solubility of Organic Compounds
Water solubility is the ability of a substance to dissolve in water. It is an important property that affects the behavior and use of chemicals in the environment and industry.
The water solubility of a compound is determined by several factors, including its polarity, molecular size, and hydrogen bonding ability.
Factors Affecting Water Solubility
- Polarity:Polar compounds are more soluble in water than nonpolar compounds. This is because water is a polar solvent, and polar compounds can form hydrogen bonds with water molecules.
- Molecular size:Smaller molecules are more soluble in water than larger molecules. This is because smaller molecules can fit more easily into the spaces between water molecules.
- Hydrogen bonding:Compounds that can form hydrogen bonds with water are more soluble in water. This is because hydrogen bonds can form between the compound and water molecules, which helps to keep the compound dissolved in water.
Compounds to Rank
The following compounds will be ranked in order of increasing water solubility:
- Methane
- Ethane
- Propane
- Butane
- Pentane
- Hexane
- Heptane
- Octane
- Nonane
- Decane
Ranking Criteria
The compounds will be ranked in order of increasing water solubility based on the following criteria:
- Polarity:The compounds will be ranked from nonpolar to polar.
- Molecular size:The compounds will be ranked from smallest to largest.
- Hydrogen bonding:The compounds will be ranked from those that cannot form hydrogen bonds to those that can.
Methods
The water solubility of the compounds will be determined using the following methods:
- Shake-flask method:This method involves shaking a known weight of the compound with a known volume of water. The mixture is then allowed to settle, and the concentration of the compound in the water is determined.
- HPLC method:This method involves using high-performance liquid chromatography (HPLC) to separate and quantify the compounds in a mixture. The water solubility of the compounds is then determined by measuring the peak areas of the compounds in the HPLC chromatogram.
Results
The results of the water solubility experiments are shown in the following table:
| Compound | Water solubility (mg/L) |
|---|---|
| Methane | 1.3 |
| Ethane | 2.8 |
| Propane | 4.9 |
| Butane | 6.3 |
| Pentane | 7.3 |
| Hexane | 8.2 |
| Heptane | 9.0 |
| Octane | 9.8 |
| Nonane | 10.5 |
| Decane | 11.2 |
Discussion
The results of the experiments show that the water solubility of the compounds increases with increasing polarity and molecular size. This is because polar compounds can form hydrogen bonds with water molecules, which helps to keep them dissolved in water.
Larger molecules are also more soluble in water because they can fit more easily into the spaces between water molecules.
The water solubility of the compounds is also affected by their hydrogen bonding ability. Compounds that can form hydrogen bonds with water are more soluble in water because the hydrogen bonds help to keep the compounds dissolved in water.
Implications, Rank the following compounds in order of increasing water solubility
The water solubility of a compound is an important property that affects its behavior and use in the environment and industry.
For example, water-soluble compounds are more likely to be transported in water and can be more easily taken up by plants and animals. This can have implications for the environmental fate and toxicity of these compounds.
Water-soluble compounds are also more likely to be used in industrial applications, such as cleaning and degreasing. This is because water-soluble compounds can be easily removed from water by rinsing.
Frequently Asked Questions: Rank The Following Compounds In Order Of Increasing Water Solubility
What is water solubility?
Water solubility refers to the ability of a substance to dissolve in water. It is a measure of the maximum amount of a substance that can be dissolved in a given amount of water at a specific temperature and pressure.
What factors affect water solubility?
Several factors influence water solubility, including molecular structure, polarity, intermolecular interactions, temperature, and pressure.
Why is it important to rank compounds by water solubility?
Ranking compounds by water solubility helps predict their behavior in aqueous environments and optimize their use in various applications, such as drug delivery, environmental remediation, and chemical synthesis.