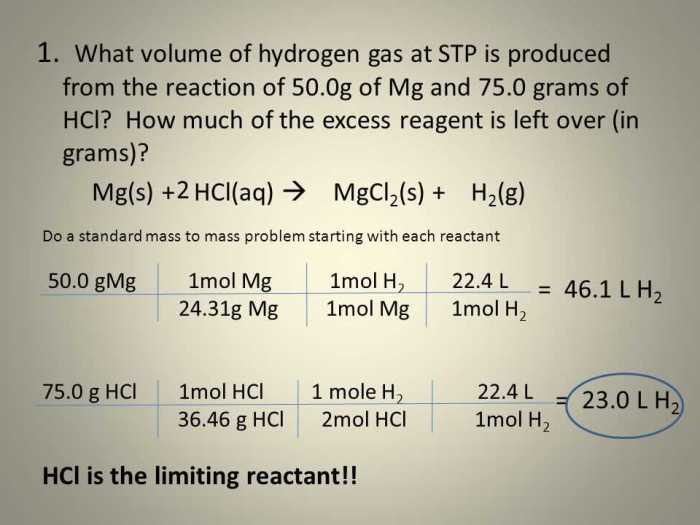
In the realm of chemical reactions, the concept of limiting and excess reactants plays a crucial role in determining the outcome and dynamics of the process. Limiting and excess reactants pogil delves into this fundamental concept, providing a comprehensive understanding of how reactant concentrations influence reaction rates, product yields, and experimental applications.
The concept of limiting reactants revolves around the notion that in a chemical reaction, one reactant is present in a quantity that limits the formation of products. This reactant, known as the limiting reactant, dictates the maximum amount of product that can be formed.
On the other hand, excess reactants are present in quantities that exceed the stoichiometric requirements for the reaction and do not limit the product formation.
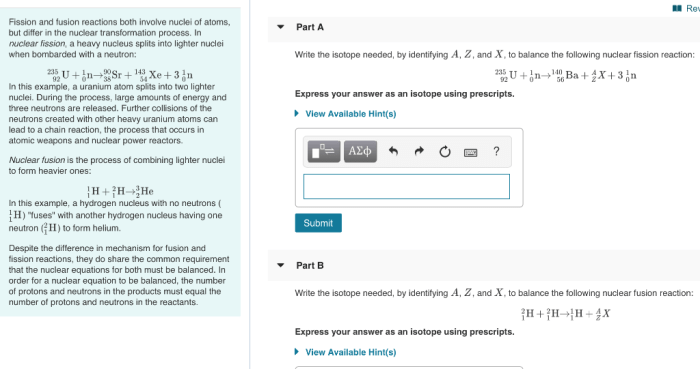
1. Limiting and Excess Reactants: Limiting And Excess Reactants Pogil
In a chemical reaction, limiting and excess reactants refer to the relative amounts of reactants present. The limiting reactant is the one that is completely consumed during the reaction, while the excess reactant is present in excess and remains after the reaction is complete.
For example, consider the reaction between hydrogen (H2) and oxygen (O2) to form water (H2O):
H2 + O2 → 2H2O
If we start with 2 moles of H2 and 1 mole of O2, the H2 will be the limiting reactant because it will be completely consumed in the reaction. The O2 will be the excess reactant because it will remain after the reaction is complete.
2. Determining the Limiting Reactant
To determine the limiting reactant in a chemical reaction, we can use stoichiometry. Stoichiometry is the study of the quantitative relationships between reactants and products in a chemical reaction.
To use stoichiometry to determine the limiting reactant, we need to:
- Write the balanced chemical equation for the reaction.
- Convert the given amounts of reactants to moles.
- Use the mole ratios from the balanced chemical equation to determine which reactant is limiting.
3. Reactant Concentrations and Reaction Rates
The concentrations of the reactants affect the reaction rate. The reaction rate is the rate at which a reaction occurs. The higher the concentration of a reactant, the faster the reaction rate.
The limiting reactant affects the reaction rate because it is the reactant that is completely consumed. Once the limiting reactant is consumed, the reaction will stop.
4. Experimental Applications
The limiting reactant concept is used in experimental chemistry to control the reaction rate. By controlling the amount of the limiting reactant, we can control the rate at which the reaction occurs.
For example, in the reaction between hydrogen and oxygen to form water, we can control the rate of the reaction by controlling the amount of hydrogen gas present.
5. Theoretical Applications, Limiting and excess reactants pogil
The limiting reactant concept has theoretical implications. The limiting reactant can be used to predict the products and yields of a chemical reaction.
For example, in the reaction between hydrogen and oxygen to form water, we can predict that the products will be water and that the yield of water will be limited by the amount of hydrogen gas present.
Q&A
What is the significance of determining the limiting reactant?
Determining the limiting reactant is crucial because it allows chemists to predict the maximum amount of product that can be formed in a reaction. It also helps in optimizing reaction conditions, such as the ratio of reactants, to achieve desired product yields.
How does the limiting reactant affect the reaction rate?
The limiting reactant controls the reaction rate because its concentration determines the rate at which the reaction proceeds. As the limiting reactant is consumed, the reaction rate slows down until it eventually stops.
What are some practical applications of the limiting reactant concept?
The limiting reactant concept finds applications in various fields, including industrial chemistry, analytical chemistry, and environmental chemistry. It is used to control reaction rates, optimize product yields, and design experiments to study chemical processes.