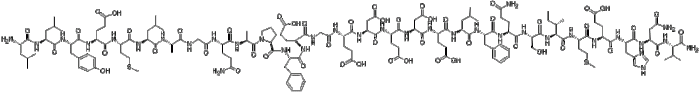
Rank the following peptides from most to least hydrophobic. – In the realm of peptide science, the concept of hydrophobicity takes center stage. Understanding the hydrophobic nature of peptides is crucial for deciphering their behavior and interactions in biological systems. This article embarks on an in-depth exploration of peptide hydrophobicity, unraveling its significance and providing a comprehensive guide to ranking peptides based on their hydrophobic properties.
Delving into the intricacies of peptide structure, we elucidate the interplay between amino acid side chains and their impact on hydrophobicity. We delve into the diverse methods employed to rank peptides based on this property, showcasing the strengths and applications of various hydrophobicity scales.
Peptide Structure and Properties
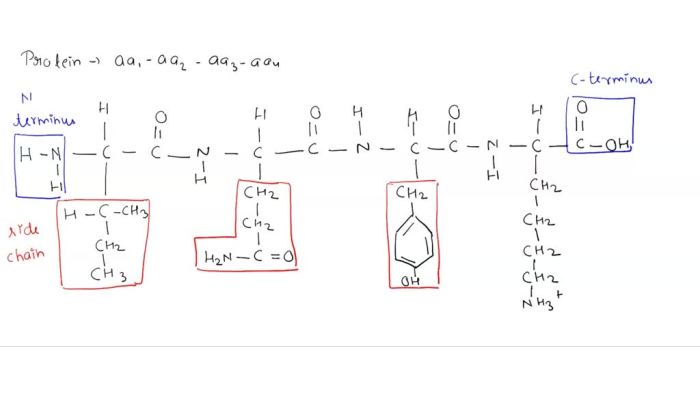
Peptides are short chains of amino acids linked by peptide bonds. The structure and properties of a peptide are determined by the sequence of amino acids in the chain. The side chains of amino acids can be either hydrophilic (water-loving) or hydrophobic (water-hating).
Hydrophobic amino acids tend to cluster together in the interior of the peptide, while hydrophilic amino acids tend to be located on the surface of the peptide.
The hydrophobicity of a peptide is a measure of its ability to dissolve in water. Hydrophobic peptides are not soluble in water, while hydrophilic peptides are soluble in water. The hydrophobicity of a peptide is determined by the balance of hydrophilic and hydrophobic amino acids in the chain.
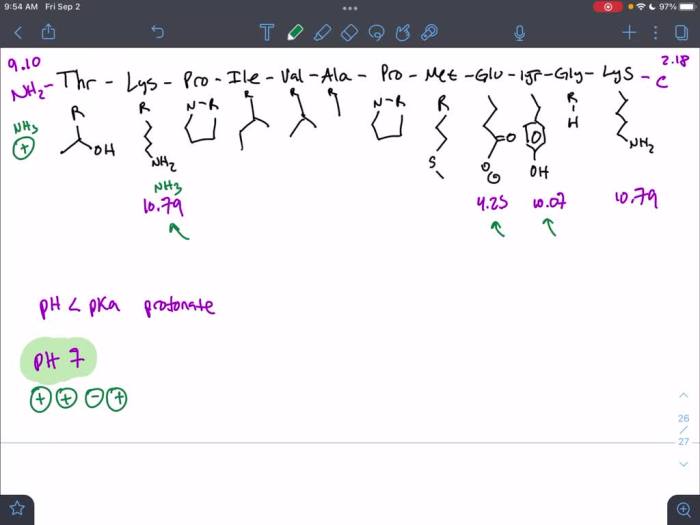
Role of Amino Acid Side Chains in Determining Hydrophobicity, Rank the following peptides from most to least hydrophobic.
The side chains of amino acids play a major role in determining the hydrophobicity of a peptide. Amino acids with nonpolar side chains are hydrophobic, while amino acids with polar side chains are hydrophilic. The following table lists the amino acids and their side chain properties:
| Amino Acid | Side Chain | Hydrophobicity |
|---|---|---|
| Alanine | -CH3 | Hydrophobic |
| Glycine | -H | Hydrophobic |
| Valine | -CH(CH3)2 | Hydrophobic |
| Leucine | -CH2CH(CH3)2 | Hydrophobic |
| Isoleucine | -CH(CH3)CH2CH3 | Hydrophobic |
| Methionine | -CH2SCH3 | Hydrophobic |
| Phenylalanine | -CH2C6H5 | Hydrophobic |
| Tryptophan | -CH2C(NH)CH2C6H4NH2 | Hydrophobic |
| Proline | -CH2CH2CH2CHCOOH | Hydrophobic |
| Serine | -CH2OH | Hydrophilic |
| Threonine | -CH(OH)CH3 | Hydrophilic |
| Cysteine | -CH2SH | Hydrophilic |
| Asparagine | -CH2CONH2 | Hydrophilic |
| Glutamine | -CH2CH2CONH2 | Hydrophilic |
| Lysine | -CH2CH2CH2CH2NH2 | Hydrophilic |
| Arginine | -CH2CH2CH2NHC(=NH)NH2 | Hydrophilic |
| Histidine | -CH2C(NH)CH2C6H4NH2 | Hydrophilic |
| Glutamic acid | -CH2CH2COOH | Hydrophilic |
| Aspartic acid | -CH2CHCOOH | Hydrophilic |
Answers to Common Questions: Rank The Following Peptides From Most To Least Hydrophobic.
What factors influence peptide hydrophobicity?
The hydrophobicity of a peptide is primarily determined by the composition and arrangement of its amino acid side chains. Hydrophobic amino acids, such as leucine and isoleucine, contribute to increased hydrophobicity, while hydrophilic amino acids, such as serine and lysine, decrease it.
How are peptides ranked based on hydrophobicity?
Various methods are used to rank peptides based on hydrophobicity. Common approaches include the Kyte-Doolittle scale, the Eisenberg scale, and the hydrophobic moment. These scales assign numerical values to each amino acid, and the sum of these values for a peptide provides an indication of its overall hydrophobicity.
What are the applications of peptide hydrophobicity?
Understanding peptide hydrophobicity is essential for a wide range of applications. It plays a crucial role in peptide interactions with membranes, protein folding, and drug design. By manipulating peptide hydrophobicity, researchers can optimize peptides for specific purposes, such as enhancing drug delivery or creating self-assembling materials.