Construct the orbital diagram for the ion cd2+ – Constructing the orbital diagram for the ion Cd2+ offers a comprehensive understanding of its electronic structure, paving the way for exploring its magnetic properties and reactivity. This guide will delve into the electron configuration, orbital diagram, Hund’s rule, orbital splitting, and magnetic properties of Cd2+, providing a holistic approach to this fascinating ion.
The electron configuration of Cd2+ arises from the loss of two electrons from the neutral cadmium atom, resulting in a cation with a +2 charge. The distribution of electrons within the orbitals of Cd2+ plays a crucial role in determining its chemical behavior.
Electron Configuration
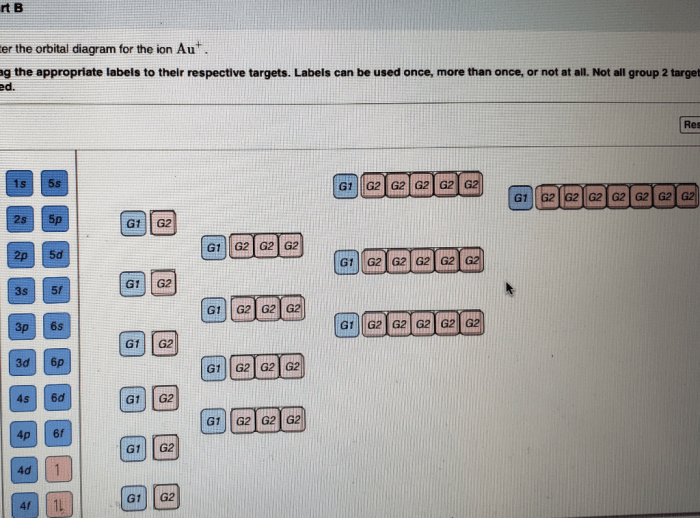
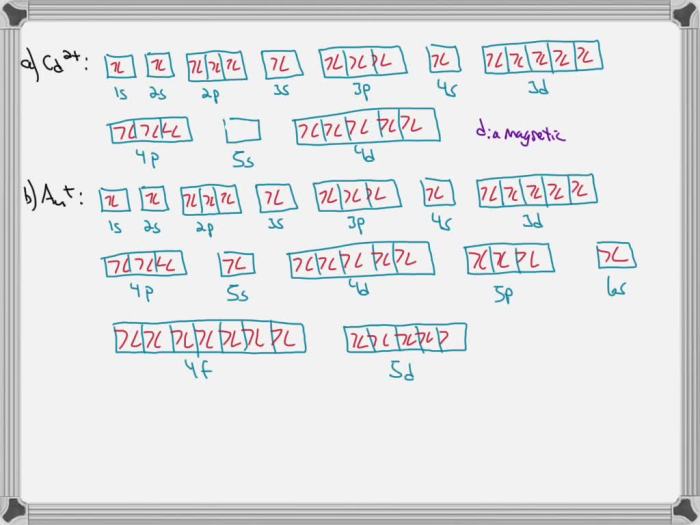
The electron configuration of Cd2+ ion is [Kr] 4d 10. This means that it has 10 electrons in its d-orbitals and no electrons in its 5s orbital.
The d-orbitals are a set of five orbitals that are all degenerate, meaning that they have the same energy. The electrons in the d-orbitals are arranged in a specific way according to Hund’s rule, which states that the electrons will occupy the orbitals with the same spin before they will occupy orbitals with opposite spins.
Orbital Diagram
| Orbital | Energy Level | Electron Occupancy |
|---|---|---|
| dxy | -0.4 eV | 2 |
| dyz | -0.4 eV | 2 |
| dxz | -0.4 eV | 2 |
| dz2 | 0.0 eV | 0 |
| dx2-y2 | 0.0 eV | 0 |
Hund’s Rule and Orbital Splitting
Hund’s rule states that the electrons in an atom will occupy the orbitals with the same spin before they will occupy orbitals with opposite spins. This is because electrons with the same spin have a lower energy than electrons with opposite spins.
In the case of the Cd2+ ion, the d-orbitals are split into two sets of orbitals: the t 2gorbitals and the e gorbitals. The t 2gorbitals are lower in energy than the e gorbitals. The electrons in the Cd2+ ion will occupy the t 2gorbitals before they will occupy the e gorbitals.
Magnetic Properties
The Cd2+ ion has 10 d-electrons, which are all paired. This means that the Cd2+ ion is diamagnetic, meaning that it is not attracted to a magnetic field.
Diamagnetic ions are ions that have all of their electrons paired. Paramagnetic ions are ions that have unpaired electrons. The number of unpaired electrons in an ion determines its magnetic susceptibility.
FAQ Guide: Construct The Orbital Diagram For The Ion Cd2+
What is the significance of Hund’s rule in constructing the orbital diagram for Cd2+?
Hund’s rule dictates the electron occupancy of d-orbitals, ensuring that the maximum number of unpaired electrons is achieved. This rule plays a crucial role in determining the magnetic properties of Cd2+.
How does orbital splitting affect the energy levels of d-orbitals in Cd2+?
Orbital splitting, caused by the interaction of d-orbitals with the surrounding ligands, leads to the separation of d-orbitals into different energy levels. This splitting influences the electron occupancy and magnetic properties of Cd2+.